Esperoct® is the result of a multi-year clinical trial shown to provide a safe and effective routine prophylaxis when administered every 4 days in adults, or twice weekly in children.
Proven safety profile.
Esperoct® safety and efficacy was studied in an extensive long-term clinical trial program for hemophilia A.
Proven safety profile.
Esperoct® safety and efficacy was studied in an extensive long-term clinical trial program for hemophilia A.

A thorough assessment.







- 0 blood clots in 270 previously treated patients
- 0 PEG-related safety concerns
- 1 previously treated patient with a high-risk gene mutation developed an inhibitor to Esperoct®a
- Similar to the reported rate in patients with severe hemophilia A
aAn 18-year-old African-American male developed an inhibitor after 93 infusion days of Esperoct®. The inhibitor rose to 13.5 Bethesda units and the patient stopped participation in the study. There was no change in efficacy, and the inhibitor eventually went away on its own (without use of immune tolerance induction therapy).
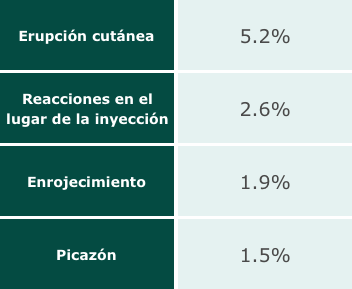
A look at side effects.

During the Esperoct® clinical trial, the most commonly reported adverse reactions were:

A product that can take the heat.

Esperoct® is the only EHL that can be stored above 86°F—all the way up to 104°F! Explore what high storage temperatures mean for you.




Answering your PEG-related questions.
PEGylation is the technology behind Esperoct’s® extended half-life formula. Learn about PEG
Answering your PEG-related questions.
PEGylation is the technology behind Esperoct’s® extended half-life formula.
Learn about PEG
Manufactured in the U.S. with strict standards.

The Novo Nordisk manufacturing principles make product safety the centerpiece of our efforts—from development to delivery.
Purity
With your safety as our guide, we commit to purity in every step of the Esperoct® process.
Reliability
You count on us to make sure your factor is available when you need it.
Consistency
Our well-established manufacturing process means you can rely on the same safe product with every infusion.
Quality
Esperoct® is made under tight control measures with quality our number one priority.

Receive email support.
Sign up to receive product information, community news, and helpful tips for living life with hemophilia.

Connect with your HCL.
Meet your Hemophilia Community Liaison (HCL)—here to help you at each step of your Esperoct® journey.


Your questions, answered.

Question: When you say that Esperoct® has a “proven safety profile,” what does that mean?
Answer: Esperoct® was studied in one of the largest and longest clinical trial programs for hemophilia A, evaluating 270 previously-treated patients over 80,000 infusion days.
Esperoct® safety was proven across 5 studies:
- 0 blood clots
- No PEG-related safety concerns
- One PTP with a high-risk gene mutation developed an inhibitor to Factor 8b
- The development of inhibitor is similar to the reported rate in patients with severe hemophilia A (0.15 per 100 patient years)c
Question: What is an “exposure day” and why is it an important safety consideration for factor VIII products?
Answer: An “exposure day” (ED) is typically considered a unit of time (1 day) during which a patient receives replacement treatment. Considering EDs during a clinical trial is helpful since inhibitors typically develop in those with severe hemophilia A after a median treatment time of 10-15 days. After 50-75 EDs, the cumulative incidence of inhibitors plateaus. However, EDs don’t tell the whole story. Other considerations that affect safety considerations include the amount of factor, frequency of infusions, the type of factor concentrate, and more.
bAn 18-year-old African American male developed an inhibitor after 93 infusion days of Esperoct®. The inhibitor rose to 13.5 Bethesda units and the patient stopped participation in the study. There was no change in efficacy, and the inhibitor eventually went away on its own (without use of immune tolerance induction therapy).
cPatient year is the patient experience under treatment of 1 year’s duration. For example, 1 patient year is equal to the experience of 2 patients for 6 months, or 12 patients for 1 month each.
![Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei]](/content/dam/biopharm/esperoct/global/esperoct-logo.png)
