Michael Norwood

“I love helping people. The patient connection is my favorite part. Finding opportunities to impact communities while challenging myself to learn something new every day.”
Long-term studies show that Esperoct® provides effective bleed control.
This could mean fewer infusions, without sacrificing protection.a
aCompared to SHL products, adults and adolescents required 50% fewer infusions when administered every other day and 40% fewer when administered 3x weekly.
Bradley has severe hemophilia A
and uses Esperoct®
Effective bleed control for all ages

bData shown are from the main phase of a study of 175 previously treated people aged 12 and older with severe hemophilia A who received Esperoct® 50 IU/kg every 4 days for 76 weeks. Median annualized bleeding rates are shown.

Factor activity between 1% and 5% is defined as moderate hemophilia
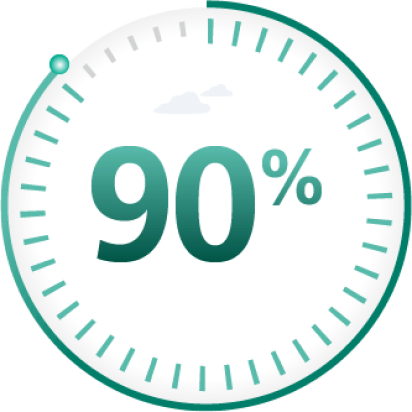
Factor activity 5% and above is defined as mild hemophilia

Maintained about 5% trough levelsf (n=53)
Trough level is where your factor is at the lowest before your next infusionc
cTrough level goal is 1% or higher for prophylaxis.
dData shown are from a study where 175 previously treated adolescents and adults with hemophilia A received routine prophylaxis with Esperoct® 50 IU/kg every four days for 76 weeks. Pre-dose factor activity (trough) levels were evaluated at follow-up visits. Mean trough levels for adolescents (aged 12-<18 years) were 2.7 IU/dL.
eSteady-state Factor FVIII activity levels were estimated in 143 adults and adolescents using pharmacokinetic (PK) modeling.
fPost hoc analyses were performed on data from a trial of patients more than 12 years of age with severe hemophilia A. Exploratory descriptive analyses of the data were used to evaluate long-term annual bleed rates and mean Factor 8 trough levels were measured over time in 61 patients who received prophylaxis every 4 days for 6 years or more. Limitations of the analyses include the absence of baseline joint status data, which limits the ability to draw conclusions regarding improvement in joint status over time. Several trough-level data were excluded if it was believed that they were elevated due to dosing to treat a recent bleed.
Bradley has severe
hemophilia A and
uses Esperoct®

0.8 overall bleeds
per yearg (N=177)

0 annual bleeds after the first year in majority of adults and adolescents who completed the entire trialh
gMedian annualized bleeding rate shown is from the main and extension phases of the pivotal clinical trial of previously treated people aged 12 and older with severe hemophilia A who received Esperoct® 50 IU/kg every days, for up to 6.6 years.
hBased on a post hoc analysis of patients who completed the entire trial (n=110) who took Esperoct® 50 IU/kg every 4 days for up to 6.6 years. Patients evaluated at Year 2 (n=103), Year 3 (n=66), Year 4 (n=62), Year 5 (n=62), and Year 6 (n=59).
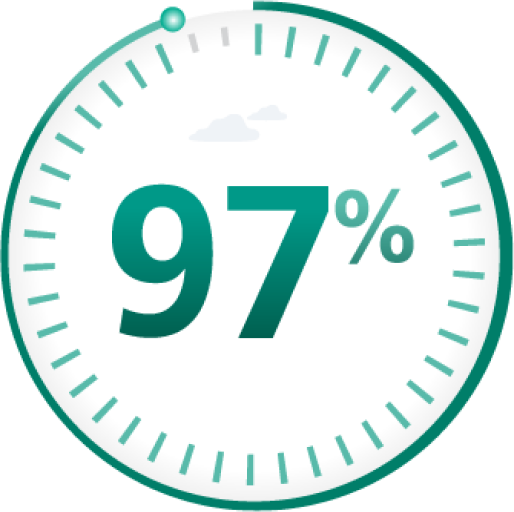
97% of bleeds controlled
with 1-2 infusionsi
iData shown are from a study where 12 adult and adolescent PTPs with severe hemophilia chose to be treated on demand and received Esperoct® for 532 bleeding episodes.
Dosing for the treatment of bleeding episodes in adults and adolescents.
For moderate bleeds, an additional dose may be administered after 24 hours
For major bleeds, additional dose(s) may be administered approximately every 24 hours
Routine prophylaxis dosing in adults/adolescents (≥12 years):
This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.
iData shown are from a study where 12 adult and adolescent PTPs with severe hemophilia chose to be treated on demand and received Esperoct® for 532 bleeding episodes.
Esperoct® achieved a 14.3 hour average half-life in childrenj

Esperoct® achieved a 14.3 hour average half-life in childrenj
That’s an 85% longer half-life compared to SHLk
jComparison to prior Factor 8 product was performed at the beginning of the study in previously treated children. The geometric mean terminal half-life in 22 children aged 0 to 11 was 14.3 hours. Esperoct® geometric mean terminal half-life was 14.7 hours in 12 children aged 0 to 5 years old and 13.8 hours in 10 children aged 6 to 11 years old.
kComparison to prior Factor 8 product was performed at the beginning of the study in previously treated children. Esperoct® half-life was 14.7 hours in 12 children aged 0 to 5 years old and 13.8 hours in 10 children aged 6 to 11 years old.
lTrough level goal is 1% or higher for prophylaxis. Trough level is when your factor is at the lowest before your next infusion.
mData shown are from a study where 34 previously treated children received routine prophylaxis with Esperoct® 60 IU/kg (50 to 75 IU/kg) twice weekly. Pre-dose factor activity (trough) levels were evaluated at follow-up visits.

2.0 overall bleeds
per yearn (N=68)

0 joint bleeds, spontaneous bleeds, traumatic bleeds per yearn

0.8 overall bleeds per yearo
N=68

Number of patients who experienced 0 annual bleeds more than doubled from Year 1 to Year 5q

100% resolution of target jointsr
nData shown are from a study of 68 previously treated children (34 aged 0–5 and 34 aged 6–11) who received an average dose of approximately 65 IU/kg twice weekly for 26 weeks. Median annualized bleeding rates are shown.
oMedian annualized bleeding rate shown is from the main and extension phases of the clinical trial in previously treated children with severe hemophilia A, for a median of 5 years.
pPost hoc analyses were performed on data from a trial of patients aged 11 and under with severe hemophilia A. Mean Factor 8 trough levels were measured over time in 54 patients who received twice-weekly prophylaxis for 5 years or more. Limitations of the analyses include the exclusion of several trough-level data if believed that they were elevated due to dosing to treat a recent bleed.
qBased on a post hoc analysis of patients who completed the entire trial who took Esperoct® 60 IU/kg (50 IU/kg to 75 IU/kg) twice weekly for up to 5 years (n=63). Approximately 32% of the patients that participated in both the main and extension phases experienced no bleeding episodes during Year 1, about 50% during Year 2, less than 50% during Year 3, 56% during Year 4, and about 70% during Year 5 had no annual bleeding episodes.
rA target joint was defined as a single joint with 3 or more bleeding episodes in 6 consecutive months. All baseline target joints reached definition of target joint resolution (if there were no bleeding episodes for 12 consecutive months) in slightly over 2 years of treatment with Esperoct®. Twelve patients with 16 documented target joints at baseline participated in the main and extension phases of the clinical trial.

Dosing for the treatment of bleeding episodes in children under 12.
For moderate bleeds an additional dose may be administered after 24 hours.
For major bleeds additional dose(s) may be administered approximately every 24 hours.
Routine prophylaxis dosing in children under 12.
This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.

270 previously
treated patients

Over 80,000
infusion days
sAn 18-year-old African American male developed an inhibitor after 93 infusion days of Esperoct®. The inhibitor rose to 13.5 Bethesda units and the patient stopped participation in the study. There was no change in efficacy, and the inhibitor eventually went away on its own (without use of immune tolerance induction therapy).
tPatient year is the patient experience under treatment of 1 year’s duration. For example, 1 patient year is equal to the experience of 2 patients for 6 months, or 12 patients for 1 month each.
“Knowing that I was going to a treatment that could fit my lifestyle was an easy decision to make.”
Vaughn has hemophilia A and uses Esperoct®
Hear more from Vaughn and other voices in the hemophilia A community.
Ready to get started?
We’ll guide you through the steps to take before your first infusion.