Frequently asked questions
Looking to learn more about Esperoct®? We’ve got the answers to your frequently asked questions.
Be sure to speak with your healthcare provider if you can’t find the answers to your questions here.
Tammy and Vaughn
have hemophilia A and
use Esperoct®
Hemophilia
Factor in the bloodstream rises and falls in patients treated prophylactically with factor. It is highest right after an infusion and lowest right before an infusion. The low-level right before infusion is called the trough level. Many factor products aim to keep trough levels at 1%. In a study of adults and adolescents who were treated with Esperoct® for prophylaxis, researchers found that extended half-life Esperoct® keeps trough levels in adults at or above 3% (moderate hemophilia) all of the time, and 5% (mild hemophilia) approximately 90% of the time, based on PK modeling.a-c
aTrough level goal is 1% or higher for prophylaxis.
bData shown are from a study where 175 previously treated adolescents and adults received routine prophylaxis with Esperoct® 50 IU/kg every 4 days for 76 weeks. Pre-dose factor activity (trough) levels were evaluated at follow-up visits. Mean trough levels for adolescents (12-<18 years) were 2.7 IU/dL.
cSteady-state factor VIII (FVIII) activity levels were estimated in 143 adults and adolescents using PK modeling.
Measuring a medicine’s half-life allows healthcare providers, patients, and caregivers to compare the factor activity level of different products by calculating the time it takes the body to metabolize or inactivate half the amount of factor.
How long factor stays in the blood, however, is only half the battle. The other consideration is the factor level. The extended half-life of Esperoct® (22 hours on average in adults) offers higher factor levels for longer compared to standard half-life products.d
dData shown are from 42 adults who received a pharmacokinetic (PK) assessment around the first Esperoct® 50 IU/kg dose.
PEGylation is a well-established technology that has been used in other treatments for many conditions including hemophilia.
About Esperoct®
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is a coagulation Factor VIII concentrate indicated for use in adults and children with hemophilia A for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
Esperoct® is administered by intravenous injection, either at a hemophilia treatment center, a healthcare provider’s office, or at home after you’ve received training. Do not administer Esperoct® yourself unless you have been shown how by a healthcare provider.
Please see Prescribing Information and Instructions for Use for complete administration information.
Esperoct® is available in six dosage strengths. Each single-dose glass vial has a color cap that will help with identification.
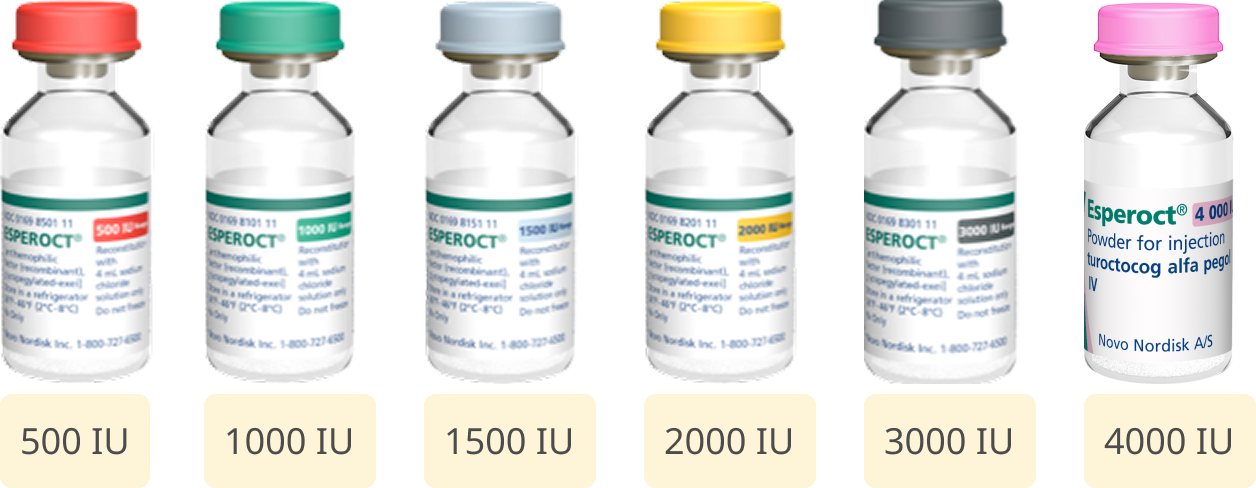
One standard dose of 65 IU/kg twice weekly is the recommended prophylaxis dose for children.
Esperoct® dosing can be individualized to meet your child’s needs. Factor 8 products may be cleared from the body faster in children under 12, which means that higher and more frequent dosing may be needed.
Your child’s healthcare provider will work with you to determine the correct dose and dosing frequency for your child.
Please see Prescribing Information and Instructions for Use for complete administration information.
One standard dose of 50 IU/kg every 4 days is the recommended prophylaxis dose for adults and adolescents.
Your healthcare provider will work with you to determine the correct dose and dosing frequency right for you.
Please see Prescribing Information and Instructions for Use for complete administration information.
Esperoct® offers on-demand bleed control that you can count on.
- A recommended dose of 40 IU/kg is used for the treatment of mild to moderate bleeding episodes in adults and adolescents.
- A recommended dose of 50 IU/kg is used for the treatment of major bleeding episodes in adults and adolescents.
Please see Prescribing Information and Instructions for Use for complete administration information.
Esperoct® offers on-demand bleed control that you can count on.
- A recommended dose of 65 IU/kg is used for the treatment of mild, moderate, and major bleeding episodes in children under 12.
Please see Prescribing Information and Instructions for Use for complete administration information.
The number of doses and length of treatment varies by both procedure and patient. Your hematologist and surgeon will work together to create the best treatment plan for your circumstances.
Please see Prescribing Information and Instructions for Use for complete administration information.
It takes two minutes for the infusion. This does not include time to set up before the infusion or time to clean up after the infusion.
Please see Prescribing Information and Instructions for Use for complete administration information.
Speak to your healthcare provider to find out more about infusion training options. Your healthcare provider may be able to offer training or direct you to a nearby hemophilia treatment center.
Do not attempt an infusion on your own unless you have been taught how by a healthcare professional.
Please see Prescribing Information and Instructions for Use for complete administration information.
Esperoct® safety and side effects
Esperoct® was studied in one of the largest and longest clinical trial programs for hemophilia A, evaluating 270 previously treated patients (PTPs) over 80,000 infusion days.
The safety of Esperoct® was proven across 5 studies, with the following results:
- 0 blood clots
- No PEG-related safety concerns
- One PTP with a high-risk gene mutation developed an inhibitor to Factor 8e
- The development of an inhibitor is similar to the reported rate in patients with severe hemophilia A (0.15 per 100 patient years)f
eAn 18-year-old African American male developed an inhibitor after 93 infusion days of Esperoct®. The inhibitor rose to 13.5 Bethesda units and the patient stopped participation in the study. There was no change in efficacy, and the inhibitor eventually went away on its own (without use of immune tolerance induction therapy)
fPatient year is the patient experience under treatment of 1 year’s duration. For example, 1 patient year is equal to the experience of 2 patients for 6 months, or 12 patients for 1 month each.
The most commonly reported adverse effects of Esperoct® include rash, redness, itching, and injection site reactions.
Storing Esperoct®
No. Esperoct® can be stored at room temperature up to 86 °F for up to 12 months and up to 104 °F for up to 3 months. Be sure to mark the date you take it out of the fridge so you can keep track. Do not return the product to the refrigerator.
See Prescribing Information for full storage details.
If a treatment becomes too hot or too cold, it could affect how it works. Prior to reconstitution, Esperoct® can be stored from 36 °F to 46 °F for up to 30 months, at room temperature up to 86 °F for up to 12 months, or up to 104 °F for up to 3 months. See Prescribing Information for complete product storage information. Be sure to mark the date you take it out of the fridge so you can keep track. Do not return the product to the refrigerator.
The high-temperature stability of Esperoct® (from 36 °F to 104 °F) helps you stay better prepared for life’s adventures or unexpected moments.
Support for you
Nobody needs to walk this path alone. Find your Novo Nordisk Rare Blood Community Liaison (RBCL) to get personalized support.
Specialty pharmacies handle complex medications like factor products. They’ll not only fulfill your factor prescription, but some will also offer services that include reconstitution and infusion training, monitoring factor levels, and communications with your healthcare team.
There are over 140 Hemophilia Treatment Centers in the U.S. These comprehensive healthcare centers not only provide a multi-disciplinary team approach to treating people with hemophilia, but many also house an in-clinic pharmacy. All of them will receive and administer treatments for their patients.
Novo Nordisk, the maker of Esperoct®, offers a few different support programs. These include the Esperoct® Co-pay Assistance Program, the Patient Assistance Program (PAP), and a free medication trial program. These programs have strict eligibility requirements. Call NovoCare® (1-888-NOVO-444, or 1-888-668-6444) to find out if you qualify.
No, NovoCare® is a free program. Our Case Managers are dedicated to providing you with the information you need to deal with insurance matters.
Stay protected from bleeds
Esperoct® can offer you the bleed protection you’re looking for.
A flexible factor
When life happens, you may want a flexible EHL product that works with your schedule.
